Orthomanual therapy as treatment for suspected thoracolumbar disc disease in dogs
Orthomanual therapy as treatment for suspected thoracolumbar disc disease in dogs
J HELLENIC VET MED SOC 2015, 66(1):31-40
Posterpresentatie Voorjaarsdagen Den Haag 2016
Samenvatting
Inleiding: Volgens de principes van de orthomanuele geneeskunde zijn standafwijkingen van de wervels geassocieerd met het ontstaan van tussenwervelschijf aandoeningen. Manuele correcties van deze standsafwijkingen zouden bij kunnen dragen aan het herstel. In deze studie is gekeken naar het effect van orthomanuele therapie (VOT) door middel van een retrospectief onderzoek in een populatie van 261 teckels die verdacht waren van thoracolumbale tussenwervelschijf problemen.
Materiaal en methoden: 261 teckels uit de medische gegevens van 2003 tot 2008 uit voldeden aan de criteria. Individuele gegevens bestonden uit signalement, geschiedenis, neurologisch en orthomanueel onderzoek voor aanvang van de behandeling, na 2 weken, 3 maanden en 6 maanden. Na een jaar werd er een telefonische enquete gehouden met de eigenaren.
Resultaten: Bij aanvang was de neurologische staat graad 1 in 115 dieren (44%), graad II in 59 dieren (23%), graad III in 27 dieren (10%), graad IV in 52 dieren (20%) en graad V in 8 dieren (3%). 2 weken na de eerste behandeling waren 111 dieren (55%) met een graad I, II of III en 2 dieren (3%) met graad IV of V verbeterd naar neurologisch normaal. Van de aanvankelijk niet ambulatoire dieren herstelde 82% naar een ambulatoire staat. Meeste dieren (78%) ondergingen een enkele orthomanuele behandeling. De meest voorkomende standsafwijkingen waren T12, T13 en L1.
Discussie: VOT gecombineerd met bench rust lijkt effectief in de behandeling van tussenwervelschijf aandoeningen bij de teckel. De resultaten van deze studie laten zien de VOT mogelijk gezien kan worden als een aanvullende therapie voor de niet chirurgische behandeling van teckels met tussenwervelschijf aandoeningen. Een prospectief gecontroleerde klinische trial is nodig om de effectiviteit verder te onderzoeken.
-----------Abstract
According to the principles of veterinary orthomanual medicine, vertebral misalignments are associated with intervertebral disc disease in dogs. Manual correction of these vertebral misalignments are presumed to contribute to successful recovery. The objective of this retrospective study was to evaluate the effects of veterinary orthomanual therapy (VOT) in 261 dachshunds with suspected thoracolumbar intervertebral disc disease (TLDD). Effect of treatment was assessed using a retrograde neurological status classification. From one clinic’s 2003-2008 medical records, 261 dachshunds with suspected TLDD met the inclusion criteria. Individual data included signalement and history, orthomanual aspects and neurological evaluations before treat- ment and at 2 weeks, 3 months and 6 months. Telephone interviews with owners were conducted one year after the initial treatment. The initial neurological status according to Griffiths ‘82 grading, was grade I in 115 animals (44%), grade II in 59 animals (23%), grade III in 27 animals (10%), grade IV in 52 animals (20%) and grade V in 8 animals (3%). Two weeks after the first treatment, 111 animals (55%) with initial grade I, II or III and two animals (3%) with initial grade IV or V had improved from their initial grade to a neurologically normal state; within 6 months of the initial treatment this full recovery was observed in 154 animals (77 %) with initial grade I, II or III and 27 animals (45%) with initial grade IV or V. Of the initially non-ambulatory dogs, 82% recovered to an ambulatory state. Of the owners, 89% evaluated the treatment as successful after 1 year. Most (78%) of the animals underwent a single VOT treatment, and the most commonly misaligned vertebrae were T12, T13 and L1. Veterinary orthomanual therapy is a conservative treatment method, which is minimally stressful for the animal and inexpensive. VOT combined with cage rest seems to be effective in treating TLDD in dachshunds. The results of this study demonstrate that veterinary orthomanual therapy might be considered an adjunct modality for the non-surgical treatment of dachshunds with intervertebral disc disease. A prospective controlled clinical trial is needed to further examine its efficacy.
Keywords
complementary medicine, dachshund, intervertebral disc disease, spinal cord injury, thoracolumbar disc disease, veterinary orthomanual medicine.
Introduction
Intervertebral disc disease (IVDD) is one of the most common neurological problems in chondrodystrophic dog breeds (Hoerlein, 1978; kornegay, 1986; Oliver 1997). In IVDD, a degeneration of the intervertebral disc occurs prior to the extrusion of the nucleus pulposus or protrusion of the annulus fibrosis. Hansen type-I IVDD is characterised by chondroid degeneration followed by extrusion of the nucleus, whereas Hansen type-II IVDD is characterised by fibroid degeneration followed by protrusion of the annulus fi (Wheeler and Sharp, 1993; Bray and Burbidge, 1998; Coates, 2000; Jaggy, 2010). The extrusion or protrusion may result in spinal cord and nerve root compression. Typically, Hansen type-I IVDD affects chondrodystrophic dogs (Oliver, 1997; Jaggy, 2010). The thoracolumbar region is predisposed to disc extrusion or protrusion (Wheeler and Sharp, 1993; Joaquim et al., 2010) which is referred to as thoracolumbar disc disease (TLDD). The manifestation of TLDD depends on the degree of damage to the spinal cord caused by primary and secondary trauma. The clinical manifestation of this disease varies widely between individuals and may include local pain, paraspinal muscle hypertonicity and pelvic limb neurological defi (Anderson et al., 1982; Lorenz et al., 2011)
Different treatment methods are available for TLDD, including movement restriction (cage rest) analgesia and anti-inflammatory drugs, physiotherapy (Levine et al., 2007; Mann et al., 2007; Lorenz et al., 2011; Ingram et al., 2013), (electro)acupuncture (Janssens and Prins, 1989; Han et al., 2010; Joaquim et al., 2010) and surgery (Hoerlein, 1978; Bitetto and kapatkin, 1989; Mckee, 1992; Sukhiani et al., 1996; Besalti et al., 2006; Mann et al., 2007; de Lahunta and Glass, 2009). Manual treatments, such as orthomanual therapy, are regularly used to treat back problems in humans and veterinary orthomanual therapy (VOT) has also been practiced for several years (Sickesz, 1986). The focus of orthomanual medicine is on nor- mal positions of components of the skeleton and symmetry in the spine (Genee et al., 2006). Manipulative techniques are applied by fixed protocols using a fast impulse (i.e. quick pressure). This force is applied on the abnormal positioned segment of the skeleton in the direction of the natural position and function (van de Veen et al., 2005).
Orthomanual medicine presumes that the degeneration of the intervertebral disc results in an instability of consecutive vertebrae. This instability can cause misalignment of vertebrae. As a consequence of this misalignment and disc degeneration, extrusion or protrusion of the nucleus pulposus or the annulus fibrosis results. The consequential pressure on the spinal cord, or trauma induced by the kinetic energy component of the extruded disc material may cause pain and neurological dysfunction. According to the principles of orthomanual medicine, a misalignment of skeletal components can cause loss of function, movement limitations and pain (van de Veen et al., 2005). It is theorised that correcting the misalignment of the vertebrae diminishes the pressure on the intervertebral disc and creates an environment that facilitates an improvement of the neurological state (As- sendelft and Lankhorst, 1998; van de Veen et al., 2005). The realignment contributes to pain relief as well. In this study, the clinical value of VOT is ret- rospectively analyzed in a population of 261 dachshunds with suspected TLDD.
MATERIALS AND METHODS
Medical records from 2003 – 2008 were searched for dachshunds that were treated with veterinary orthomanual medicine (VOM) for suspected TLDD. Patients with concurrent orthopaedic, systemic or neurological disease were excluded from the study. Patients with neck pain, lower back pain, and/ or vertebral misalignments other than in the thoracolumbar region were also excluded. Furthermore, cases were excluded when complete medical record data was not available.
Suspicion of TLDD was based on the patients’ typical history and clinical findings (Wheeler, 1995; Oliver, 1997; Levine et al., 2007). The dogs were examined by one veterinarian trained in VOM. During neurological examination the following items were evaluated: the ability to walk, spinal pain, posterior paresis or paralysis, proprioception, spinal reflexes, superficial and deep nociception and bladder function and control. Neurological grading according to a modifi Griffi ‘82 scale (Wheeler and Sharp, 1993) was used. In short this grading comprises grade I only pain, grade II posterior paresis with absent proprioception, grade III paralysis with absent proprioception, grade IV paralysis with absent proprioception and absent bladder control, grade V paralysis with absent proprioception, absent bladder control and absent deep nociception. Neurological grades were evaluated before the treatment, at mean 14 days (range: 12 – 16 days), at mean 104 days (range: 89 – 119 days) and at 6 months after the initial visit.
Data collection consisted of age, gender, weight, disease onset, period before first VOM treatment, general examination, initial and repeated neurological examination, medication prior to referral, orthomanual aspects (misaligned vertebrae), evaluation of the animal’s neurological performance by the owner 1 year after the first treatment and the number of examinations and treatments. The clinical results were expressed as a difference in neurological grade compared to day 0, using a retrograde neurological status classification (Table 1). This classification dis- criminates between a completely normal neurological status and minor residual neurological abnormalities, such as delay in proprioceptive positioning or slight paresis when, for instance, lifting a hind leg to urinate in males. One year follow-up interviews were conducted by telephone, with the main criterion being whether the animal exhibited normal physical abilities.
Veterinary orthomanual therapy
In VOM special attention is given to interpretation of vertebral positions, such as (i) symmetry and vertebrae alignment (by palpation and visual observation of positions of the thumbs), (ii) local paraspinal muscle tone, (iii) whether muscle atrophy is present. Furthermore, attention is paid to hyperaesthesia. Palpation is performed along the entire vertebral column from the tail to the head by placing the thumbs on either side of the vertebrae dorsal spinous process (Figs 1 and 2). An assistant, using both hands, fully supports the dog and positions it squarely in front of the veterinarian. The condition of the animal is con- sidered abnormal when the vertebrae are misaligned along the longitudinal axis or with deviations in the ventrodorsal and lateral (in VOM termed dextro-sinistral) axes. In addition, the position of a vertebra is considered misaligned if the transverse processes of the vertebrae are not parallel to each other, with difference in the level or muscle tone on either side of the vertebra. Several misaligned vertebral positions could be observed in the dogs, including 1. tipper (ventro position): rotation of the vertebra on the longitudinal axis with slight rotation in the sagittal plane to the homolateral side; 2. tumbler: rotation of the vertebra on the sagittal plane with a slight rotation to the homolateral side; 3. slight dorsal dislocation: the frontal part of the vertebra is slightly elevated in the transversal plane; and 4. lateral translation: misalignment along the lateral axis (Fig. 3).
The aim of VOT is to correct misaligned vertebral positions. To do this the veterinarian first slightly elevates the vertebral body with one hand and then imparts an impulse to the spinous process with the thumb of the other hand, thereby pushing it into the correct position. Misaligned vertebrae generally require little force to be pushed back into their original positions. All of the misaligned vertebrae are repositioned in a single session. After the first treatment all medications are discontinued if further analgesia is not required and the animals are confined to cage rest for two weeks (Wheeler and Sharp, 1993). After the period of cage rest limited exercise is gradually initiated by the owner according to an individual VOM revalidation protocol. Movement restriction (cage rest) and good care (nursing) are also necessary for neurological improvement (Simpson, 1992; Levine et al., 2007). General and neurological examinations are repeated during the two follow-up visits, and neurological status was graded again. Vertebral misalignments are diagnosed by inspection and palpation during the orthomanual examination of each follow-up. Any misaligned vertebrae, if present, are repositioned.
Table 1. recovery classification in retrograde neurological status.
| Score | Definition |
|---|---|
| 0 | Clinically and neurologically normal,unremarkable neurological examination |
| I | Ambulatory with slight residual ataxia or proprioceptive delay |
| II | Ambulatory but paretic, proprioceptive deficits |
| III | Non-ambulatory: posterior paralysis, intact bladder control |
| IV | Non-ambulatory: posterior paralysis, absent bladder control |
| V | Non-ambulatory: posterior paralysis, absent bladder control, absent deep nociception |
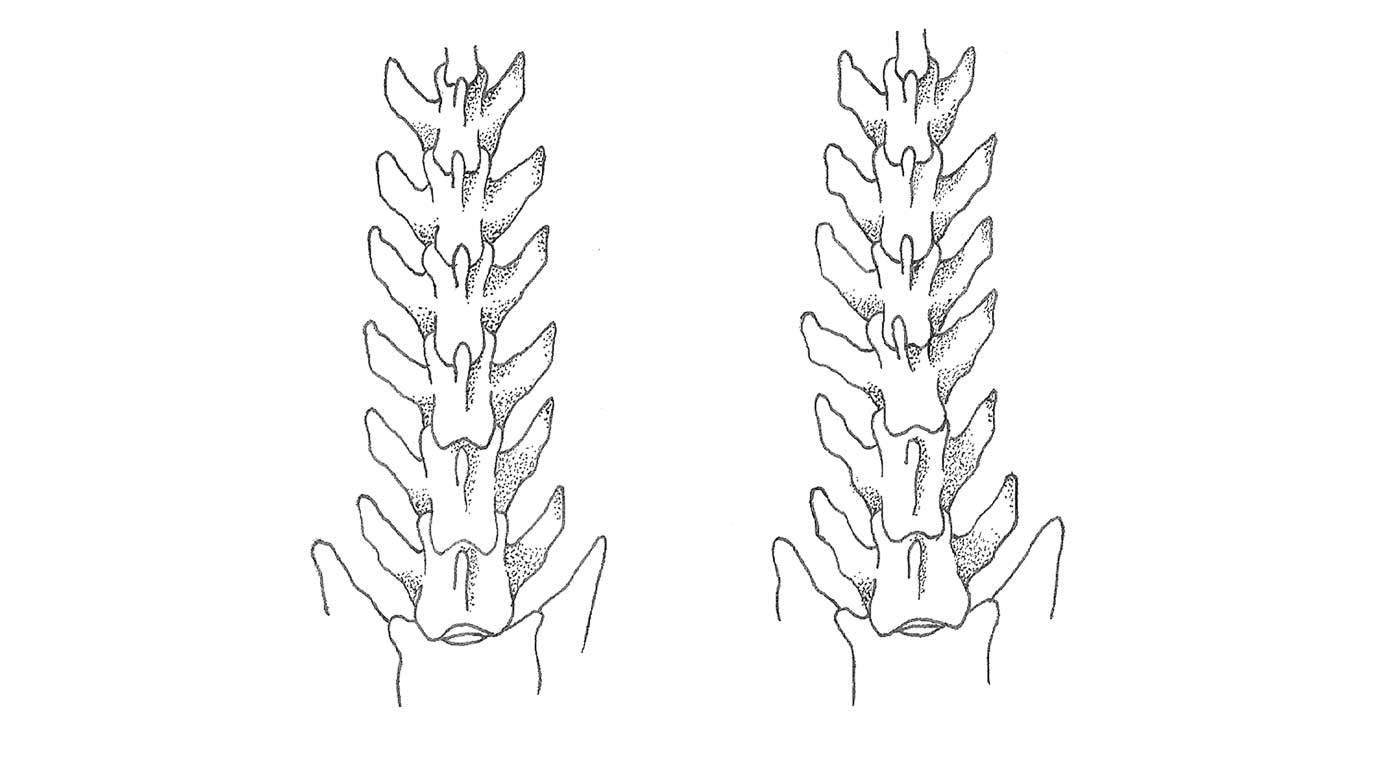
Figure 1. Normal alignment of the vertebral column (left) and misalignment of the 5th lumbar vertebra in the frontal plane.
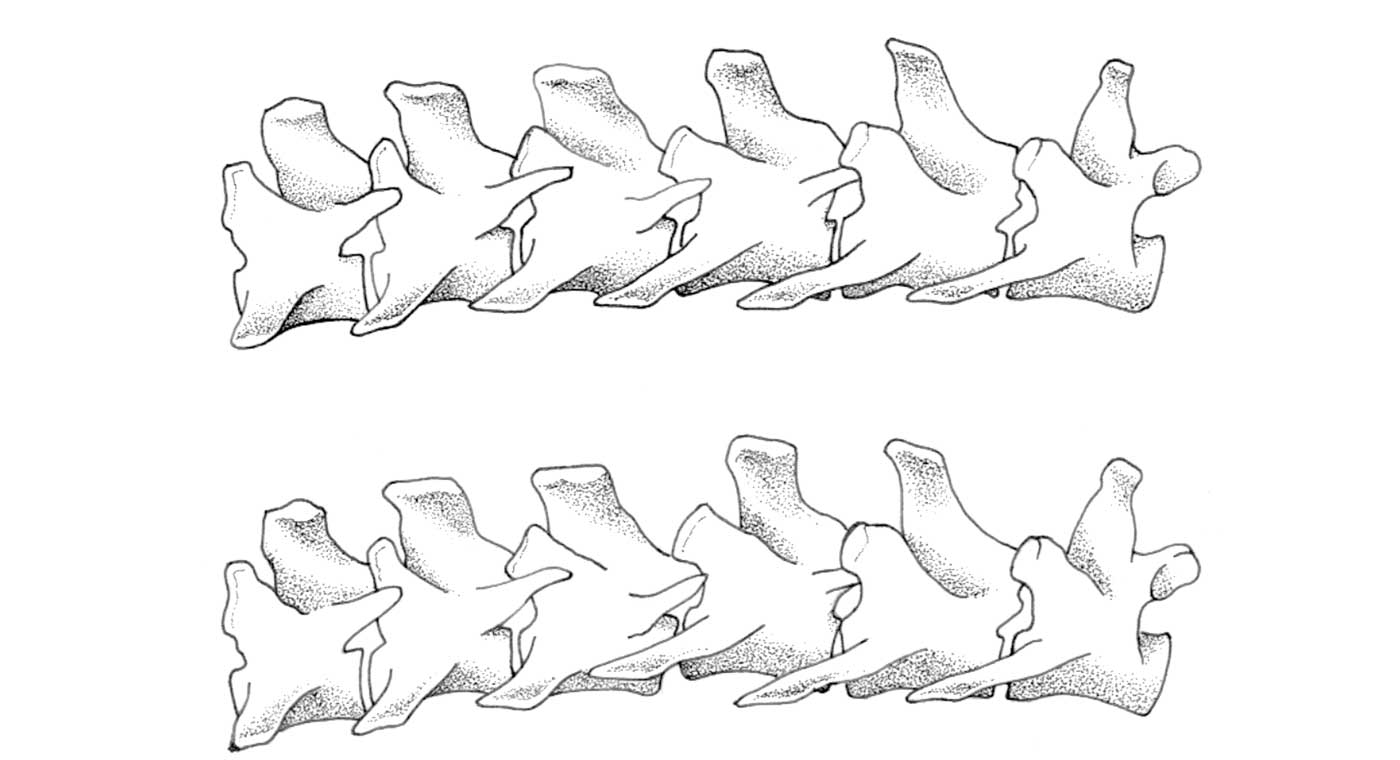
Figure 2. Normal alignment of the vertebral column (upper illustration) and slight craniodorsal displacement or misalignment (lower illustration). The caudal site of the animal is on the right in the figure.
Data analysis
We calculated the median and range values for age and weight and determined prevalence of gender. Animals were sorted into groups according to their initial neurological grade. Misaligned vertebrae were determined during every visit and the type of misalignment was documented. Neurological change after each treatment was documented at each visit. The evaluation at the 1 year telephone evaluation involved placement into one of the following categories per group of initial neurological diagnosis: good, unsuccessful, euthanasia for another reason (Euth. oth.) or unknown. The evaluation was considered “good” if the animal was pain free and exhibited normal physical abilities in and around the house. The evaluation was considered unsuccessful if the animal was not normal in function, had relapsed, was referred to surgery because of unsatisfactory improvement or be- cause it remained paralytic or was euthanized because of the severe neurological state.
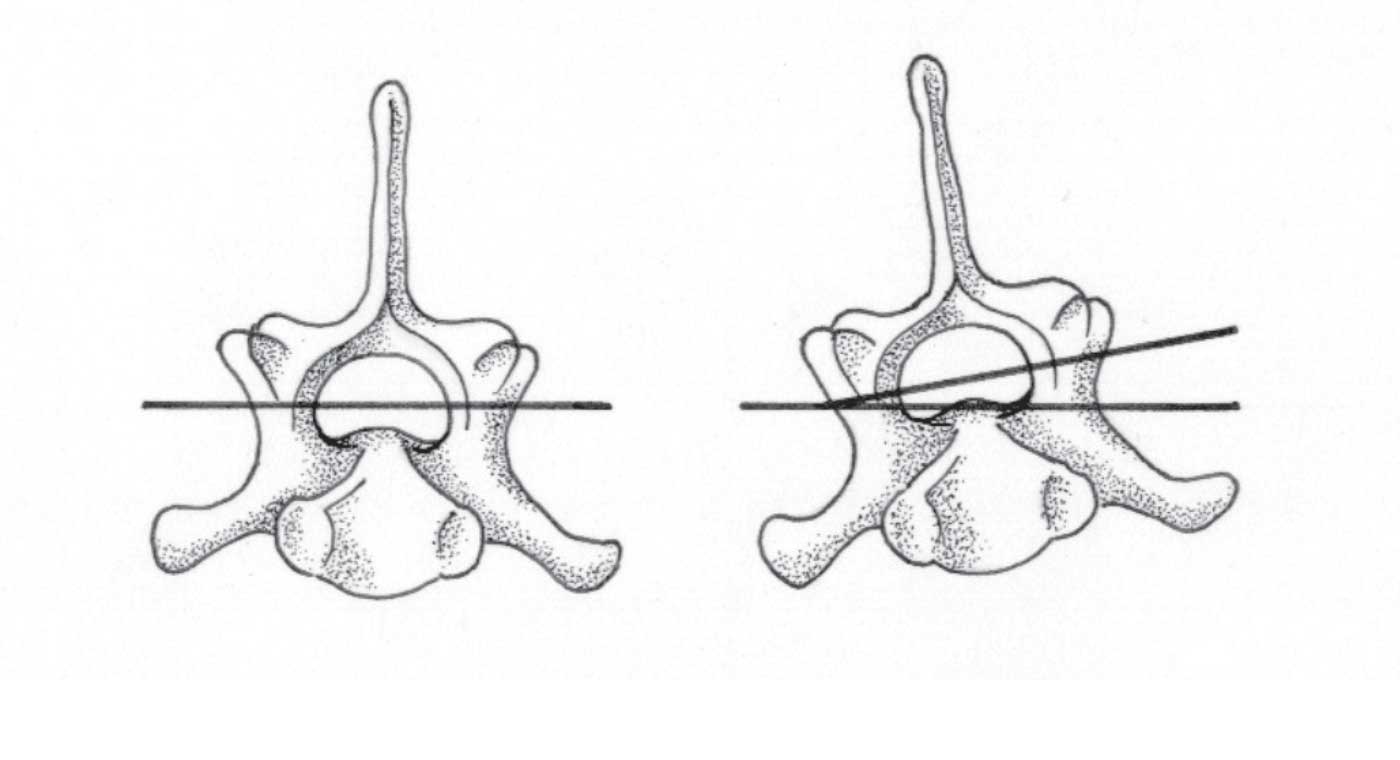
Figure 3. Normal alignment of a vertebra (left) and a tipper
RESULTS
In total, 261 Dachshunds were included; 96 (37%) were intact male, 48 (18%) intact female, 39 (15%) castrated male and 78 (30%) neutered female dogs. The mean age was 6.4 years (range: 1 to 15 years), and the mean weight was 8.2 kg (range: 2.8 to 18 kg). The initial neurological status was diagnosed as grade I in 115 animals (44%), grade II in 59 animals (23%), grade III in 27 animals (10%), grade IV in 52 animals (20%) and grade V in 8 animals (3%). 105 Out of 261 dogs (40%) had symptoms of acute onset and 156 (60%) of chronic onset. Prior to VOT, 94% of the animals were medicated before (NSAIDs or corticosteroids). In this patient population, the most commonly misaligned vertebrae were T12, T13 and L1. The most commonly detected type of misalignment was the tip- per (ventroposition).
Two weeks after the first treatment 261 (100%) dogs revisited. 111 of the 201 animals (55%) with initial grade I, II or III exhibited an improvement from their initial neurological grade to normal. A majority (58%) of the animals initially diagnosed as grade II exhibited an improvement of one grade, whereas 44% of animals initially diagnosed as grade III exhibited an improvement of two grades. Furthermore, 2 of the 60 animals (3%) with initial grade IV or V exhibited an improvement from their initial neurological grade to normal. Of the animals initially diagnosed as grade IV, 35% exhibited an improvement of one grade, whereas half of the animals (50%) initially diagnosed as grade V exhibited an improvement of two grades (Table 2). Two patients showed deterioration and were subsequently referred for further diagnostics and surgery.
At three months (mean 104 days) 197 (75%) animals returned for follow-up. 116 Out of the 145 animals (80%) that revisited with initial grade I, II or III exhibited an improvement from their initial neurological grade to normal. A majority (71%) of the animals initially diagnosed as grade II and 46% of initially graded III exhibited an improvement of two grades. Furthermore, 19 out of the 52 animals that revisited (37%) examined with initial grade IV or V, exhibited an improvement to neurologically normal. 43% Of the animals initially diagnosed as grade IV exhibited an improvement of three grades, and 43% of animals initially diagnosed as grade V exhibited an improvement of two grades (Table 3).
At the six months follow-up 81% of all animals had recovered to a pain free and ambulatory state with 28% exhibiting minor residual ataxia (grade I) and 53% considered completely normal (grade 0) . Of the animals initially diagnosed as grade IV or V, 98% improved at least 1 neurological grade. The 2 animals that had deteriorated and were referred for surgery at 2 weeks have been included in the 6 month table for sake of completeness (Table 4).
In the 1 year telephone evaluation 95% of the owners responded. 181 animals (90 %) with initial grade I, II or III were evaluated by the owner as “good”. Furthermore, 52 animals (87%) with initial grade IV or V were evaluated as “good” (Table 5). A single patient was euthanized for reasons unrelated to spinal injury in the interval between the last visit and telephone contact for owner evaluation. Most of the animals (203, i.e. 78%) underwent a single VOT treatment during the six-month study period (Table 6). The following factors were significantly associated with and may have negatively influenced neurological outcome: 1. insufficient cage rest, 2. prior corticosteroid therapy (Levine et al., 2007) and 3. excess body weight. The following factors may have positively influenced the neurological outcome: 1. cage rest 2. prior corticosteroid therapy.
Table 2. Changes in neurological scoring at two-week follow-up visit.
| Start score | |||||
|---|---|---|---|---|---|
| Final score | I | II | III | IV | V |
| 0 | 87(67%) | 19(32%) | 5(19%) | 2(4%) | |
| I | 28(24%) | 34(58%) | 12(44%) | 9(17%) | |
| II | 4(7%) | 4(15%) | 17(33%) | 1(13%) | |
| III | 1(2%)1 | 6(22%) | 18(35%) | 4(50%) | |
| IV | 1(2%) | 6(12%) | 2(25%) | ||
| V | 1(13%) | ||||
| Total | 115 | 59 | 27 | 52 | 8 |
Numbers in red colour indicate deterioration.
Table 3. Changes in neurological scoring at three-month follow-up visit.
| Final score | I | II | III | IV | V |
| 0 | 75(96%) | 32(71%) | 9(41%) | 18(40%) | 1(14%) |
| I | 2(3%) | 13(29%) | 10(46%) | 19(42%) | 1(14%) |
| II | 1(1%) | 1(5%) | 6(13%) | 2(29%) | |
| III | 2(9%) | 2(4%) | 3(43%) | 18(35%) | 4(50%) |
| IV | |||||
| V | |||||
| Total | 78 | 43 | 22 | 45 | 7 |
Not all dogs were reassessed at the three-month evaluation.
Table 4. Changes in neurological scoring at six-month follow-up visit.
| Final score | I | II | III | IV | V |
| 0 | 106(92%) | 38(64%) | 10(37%) | 25(48%) | 2(25%) |
| I | 9(8%) | 17(29%) | 13(48%) | 17(33%) | 2(25%) |
| II | 2(4%) | 1(4%) | 7(14%) | 1(13%) | |
| III | 1(2%) | 3(11%) | 1(2%) | 3(38%) | |
| IV | 1(2%) | 2(4%) | |||
| V | |||||
| Total | 115 | 59 | 27 | 52 | 8 |
Numbers in red colour indicate deterioration.
Table 5. Results of owner evaluation of animal condition at one-year telephone follow-up.
| Start score | |||||
|---|---|---|---|---|---|
| Evaluation | I | II | III | IV | V |
| Good | 109(95%) | 54(92%) | 18(67%) | 47(90%) | 5(63%) |
| Unsuccessful | 1(1%) | 4(7%) | 4(15%) | 2(4%) | 3(38%) |
| Euth other | 1(1%) | ||||
| No answer | 4(4%) | 1(2%) | 5(19%) | 3(6%) | |
| Total | 115 | 59 | 27 | 52 | 8 |
Table 6. Total number of orthomanual treatments in a six-month period.
| Start score | |||||
|---|---|---|---|---|---|
| No. of treatments | I | II | III | IV | V |
| 1 | 95(83%) | 48(81%) | 19(70%) | 36(69%) | 5(63%) |
| 2 | 19(17%) | 11(19%) | 7(26%) | 5(10%) | 2(25%) |
| 3 | 1(1%) | 1(4%) | 10(19%) | 1(13%) | |
| 4 | 1(2%) | ||||
| Total | 115 | 59 | 27 | 52 | 8 |
Discussion
A preliminary diagnosis of TLDD is often made based on the animal’s history together with information on breed, age and clinical signs (Wheeler, 1995; Oliver, 1997). Additional diagnostics are required to confirm the diagnosis. Normally myelography, computed tomography (CT) or magnetic resonance imaging (MRI) are performed but they tend to be reserved if surgical management is required.
Dachshunds are predisposed to develop TLDD, making them a suitable model to study the effects of VOT in TLDD (Goggin et al., 1970; Ball et al., 1982; Olby et al., 2004). In this study, the mean age of the affected animals was 6.4 years, which is in accordance with previous findings (Mckee, 1992; Scott, 1997; Nečas, 1999). The most commonly misaligned vertebrae observed in our study were T12, T13 and L1. According several studies, most disc lesions in chondrodystrophic dog breeds occur between T12 and T13, and T13 and L1 (Gambardel- la, 1980; Mckee, 1992; Scott, 1997; Brisson et al., 2004; Tanaka et al., 2004; Levine et al., 2009).
Orthomanual therapy in dogs with TLDD is presumed to create a condition that facilitates improvement of the neurological state by relieving pressure on the intervertebral disc and spinal cord (Assendelft and Lankhorst, 1998; van de Veen et al., 2005). The addition of cage rest to this therapy allows the animal’s body to resorb a part of the extruded disc material and to strengthen the annulus fibrosus, and reduces the risk of further nucleus pulposa extrusion. Cage rest also decreases the incidence of self-trauma due to incoordination (Simpson, 1992; Mochida et al., 1998; Doita et al., 2001; Levine et al., 2007).
One study of medical conservative treatment reported successful treatment of 100% of animals with hyperpathia with or without mild neurologic deficits (i.e., grade I or II in our neurological grading) (Mann et al., 2007). A success rate of 54.7% in a patient group with initial neurological grade I to V was reported in another study of medical conservative treatment, with dogs that were completely nor- mal and without recurrence of clinical signs (Levine et al., 2007). In a third study, success rates of 100%, 84%, 100%, 50% and 7% were reported in respectively initial grade I, II, III, IV and V, with residual paresis (i.e., at least grade I in our neurological grading) in 13% of the animals (Olby et al., 2004). In our study, patients with residual paresis were graded I-II and not considered fully recovered, which makes outcome percentages difficult to compare. In this light, success rates according to the retrograde classification for initial grades I-V were 92%, 64%, 37%, 48% and 25% respectively. When not taking residual paresis into account however, success rates were 100%, 97%, 89%, 94% and 63% respectively.
For initially non-ambulatory patients, the ability to walk voluntarily after treatment and the time to ambulation are the criteria commonly used to discern between a successful and unsuccessful outcome. Recovery rates including animals with residual pare- sis after decompressive surgery in non-ambulatory dogs with intact deep nociception (i.e. grade III and IV in our study) vary between 86% and 96% (Gam- bardella, 1980; Scott, 1997; Nečas, 1999; Davis and Brown, 2002; Ferreira et al., 2002; Brisson et al., 2004; ruddle et al., 2006; Bush et al., 2007). In this study 78% of the initial grade III and 54% of the grade IV animals were ambulatory within 2 weeks, and respectively 89% and 94% within 6 months. However, 44% of grade III and IV animals were con- sidered completely recovered without any residual paresis.
Absent deep nociception (grade V) is often associated with severe spinal cord damage and worse prognosis (Henke et al., 2013). reported percentages of functional recovery after surgery in dogs without deep nociception ranges from 38% to 76% (Aikawa et al., 2012). In our study 63% of the initial grade V dogs recovered to an ambulatory state and were considered successfully treated by the corresponding 63% of owners at the 1 year evaluation. Practical experience indicates that reoccurrence of vertebral misalignments, once treated, is rare. This is in conclusion with a single treatment that was needed for the majority of patients, as shown in table 6. The neurological grade at onset is positively correlated with the number of orthomanual treatments, which suggests that the likeliness for vertebral misalignment to reoccur might be related to the severity of spinal cord compression.
The lack of diagnosis confirmation by medical imaging is a limitation of this study and it is possible that some animals had other conditions that led to the same symptoms. Also, due to lack of diagnostic imaging, no distinction was made between HNP type 1 and type 2 and this may account for some variability in outcome. Other limitations are the relatively small group of initially graded V dogs, outcomes based on telephone interviews with owners that are prone to information bias, and its retrospective nature. Due to the lack of a control group, it remains unclear what the natural course of the disease would have been, and if and to what extent VOT contributed to recovery. It is well known that many ambulatory dogs with thoracolumbar spinal lesions (grade I-II) recover with medical management and cage rest alone (Ingram et al., 2013). However, recovery rates for non-ambulatory patients (grade III-V) with conservative management alone are considerably lower in proportion to the initial neurological grade, and spontaneous recovery from grade IV and V lesions is unlikely (Lorenz et al., 2011; Wheeler and Sharp, 1993). Many dogs in our study had already been treated unsuccessfully with steroids, NSAIDs and cage rest, and improved only after VOT treatment. Also, recovery rates after VOT for grade III-V dogs were good and similar to those reported for surgical treatment.
CONCLUDING REMARKS
In this study a retrograde neurological classification system was introduced with the aim of creating uniformity in describing and quantifying neurological recovery from spinal cord injury. Veterinary orthomanual therapy combined with cage rest seems to be effective in treating TLDD in dachshunds and might be considered an acceptable form of non-surgical treatment. Furthermore, VOT is minimally stressful for the animal, inexpensive and non-invasive. Success rates from this retrospective study are comparable with those reported for decompressive surgery, and long-term owner evaluations were excellent. No recidives or complications were reported in the timespan of 1 year.
This is the first study to describe the effects of orthomanual therapy in dogs with suspected intervertebral disc disease. Further research is required to study other breeds, larger groups of grades III to V, treatment mechanism and the correlation between diagnostic fi and VOM clinical fi. A prospective controlled clinical trial is the next logical step.
ACKNOWLEDGEMENTS
The authors would like to thank Henk Halsema for help with the figures, and Prof. Dr. Andre Jaggy, Dr. Luc Janssens, Dr. Clare rusbridge and Dr. Franck Forterre for their advice.
CONFLICT OF INTEREST STATEMENT
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
REFERENCES
Aikawa T, Fujita H, kanazono S, Shibata M, Yoshigae Y (2012) Long-term neurologic outcome of hemilaminectomy and disk fenestration for treatment of dogs with thoracolumbar intervertebral disk herniation: 831 cases (2000- 2007). J Am Vet Med Assoc. 241:1617-1626.
Anderson Dk, Means ED, Waters Tr, Green ES (1982) Microvascular perfusion and metabolism in injured spinal cord after methylprednisolone treatment. J Neurosurg 56:106-113.
Assendelft WJJ, Lankhorst GJ (1998) Effectiviteit van manipulatieve therapie bij lage rugpijn: geen uitsluitsel in systematische literatuuroverzichten en behandelrichtlijnen. Nederl Tijdsc Geneesk 142:684-688.
Ball Mu, McGuire JA, Swaim SF, Hoerlein BF (1982) Patterns of occurence of disk disease among registered dachshunds. J Am Vet Med Assoc 180:519-522.
Besalti O, Peckan Z, Sirin YS, Erbas G (2006) Magnetic resonance imaging findings in dogs with thoracolumbar intervertebral disk disease: 69 cases (1997-2005). J Am Vet med Assoc 228:902-908.
Bitetto WV, kapatkin AS (1989) Intraoperative problems associated with intervertebral disc disease. Probl Vet Med 1:434-444. Bray JP, Burbidge HM (1998) The canine intervertebral disk. Part two: degenerative changes-non chondrodystrophoid versus chondrodystrophic disks. J Am Anim Hosp Assoc 34:135-144.
Brisson BA, Moffatt SL, Swayne SL, Parent JM (2004) Recurrence of thoracolumbar intervertebral disk extrusion in chondrodystrophic dogs after surgical decompression with or without prophylactic fenestration: 265 cases (1995-1999). J Am Vet Med Assoc 224:1808-1814.
Bush WW, Tiches DM, kamprad C (2007) Functional outcome following hemilaminectomy without methylprednisolone sodium succinate for acute thoracolumbar disk disease in 51 non- ambulatory dogs. J Vet Emerg Crit Care 17:72-76.
Coates Jr (2000) Intervertebral disk disease. Vet Clin N Am Small Anim Pract 30:77-110.
Davis GJ, Brown DC (2002) Prognostic indicators for time to ambulation after surgical decompression in nonambulatory dogs with acute thoracolumbar disk extrusions: 112 cases. Vet Surg 31:513-518.
De Lahunta A, Glass EN (2009) Small animal spinal cord disease. In: (eds: De Lahunta A, Glass EN) Veterinary Neuroanatomy and Clinical Neurology, 3rd edn. Elsevier, St. Louis, pp. 243-265.
Doita M, kanatani T, Ozaki T, Matsui N, kurosaka M, Yoshiya S (2001) Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine 26:1522-1527.
Ferreira AJA, Correia JHD, Jaggy A (2002) Thoracolumbar disc disease in 71 paraplegic dogs: influence of rate of onset and duration of clinical signs on treatment results. J Small Anim Pract 43:158-163.
Gambardella PC (1980) Dorsal decompressive laminectomy for treatment of thoracolumbar disc disease in dogs: a retrospective study of 98 cases. Vet Surg 9:24-26.
Genee T, van de Bunt A, de Bruijne, JJ (2006) De standafwijkingen. In: (eds: Genee T, van de Bunt A, de Bruijne JJ et al.) Orthomanuele Geneeskunde, 1st edn. Nederlandse Vereniging Orthomanuele Geneeskunde, Amsterdam, pp. 54-93.
Goggin JE, Li AS, Franti CE (1970) Canine intervertebral disc disease: characterization by age, sex, breed, and anatomic site of involvement. Am J Vet Res 31:1687-1692.
Han HJ, Yoon HY, Kim JY, Jang HY, Lee B, Choi SH, Jeong SW (2010) Clinical effect of additional electroacupuncture on thoracolumbar intervertebral disc herniation in 80 paraplegic dogs. Am J Chin Med. 38:1015-1025.
Henke D, Vandevelde M, Doherr MG, Stöckli M, Forterre F (2013) Correlations between severity of clinical signs and histopathological changes in 60 dogs with spinal cord injury associated with thoracolumbar intervertebral disc disease. Vet J 198:70-75.
Hoerlein BF (1978) Invertebral disks. In: (ed: Hoerlein BF) Canine neurology: diagnosis and treatment, 3rd edn. Saunders, Philadelphia, pp. 470-560.
Hoerlein BF (1978) The status of various intervertebral disc surgeries for the dog in 1978. J Am Anim Hosp Assoc 14:563-570. Ingram EA, Kale DC, Balfour rJ (2013) Hemilaminectomy thoracolumbar Hansen Type I intervertebral disk disease in ambulatory dogs with or without neurologic defi its: 39 cases (2008-2010). Vet Surg 42:924-931.
Jaggy A (2010) Spinal cord. In: (ed: Jaggy A) Small animal neurology, 1st edn. Schlütersche, Hannover, pp. 351-356. Janssen LAA, Prins EMD (1989) Treatment of thoracolumbar disc disease in dogs by means of acupuncture: a comparison of two techniques. J Am Anim Hosp Assoc 25:169-174.
Joaquim JG, Luna SP, Brondani JT, Torelli SR, Rahal SC, de Paula Freitas F (2010) Comparison of decompressive surgery, electroacupuncture, and decompressive surgery followed by electroacupuncture for the treatment of dogs with intervertebral disk disease with long-standing severe neurologic deficits. J Am Vet Med Assoc. 236:1225-1229.
kornegay JN (1986) Invertebral disk disease. In: (eds: komegay JN) Neurologic disorders – contemporary issues in small animal practice, 1st edn. Church Livingstone, New York, pp. 21-39.
Levine JM, Fosgate GT, Chen AV, rushing r, Nghiem PP, Platt Sr, Bagley rS, kent M, Hicks DG, Young BD, Schatzberg SJ (2009) Magnetic resonance imaging in dogs with neurologic impairment due to acute thoracic and lumbar intervertebral disk herniation. J Vet Intern Med 23:1220-1226.
Levine JM, Levine GJ, Johnson SI, kerwin SC, Hettlich BF, Fosgate GT (2007) Evaluation of the success of medical management for presumptive thoracolumbar intervertebral disk herniation in dogs. Vet Surg 36:482-491.
Lorenz MD, Coates Jr, kent M (2011) Confirming a Diagnosis. In: (eds: Lorenz MD, Coates Jr, kent M) Handbook of veterinary neurology, 5th edn. Saunders/Elsevier, St. Louis, pp. 75-77.
Mann FA, Wagner-Mann CC, Dunphy ED (2007) recurrence rate of presumed thoracolumbar intervertebral disc disease in ambulatory dogs with spinal hyperpathia treated with anti- inflammatory drugs: 78 cases (1997 – 2000). J Vet Emerg Crit Care 17:53-60.
Mckee WM (1992) A comparison of hemilaminectomy (with concomitant disc fenestration) and dorsal laminectomy for the treatment of thoracolumbar disc protrusion in dogs. Vet Rec 30:296-300.
Mochida K, Komori H, Okawa A, Muneta T, Haro H, Shinomiya k (1998) regression of cervical disc herniation observed on magnetic resonance images. Spine 23:990-995.
Nečas A (1999) Clinical aspects of surgical treatment of thoracolumbar disc disease in dogs. A retrospective study of 300 cases. Acta Vet 68:121-130.
Olby N, Harris T, Burr J, Muñana k, Sharp N, keene B (2004) Recovery of pelvic limb function in dogs following acute intervertebral disc herniations. J Neurotr 21:49-59.
Oliver JE (1997) Pelvic limb paresis, paralysis, or ataxia. In: (eds: Oliver JE, kornegay JN, Lorenz MD) Handbook of veterinary neurology, 3rd edn. Saunders, Philadelphia, pp. 133-138.
ruddle TL, Allen DA, Schertel Er, Barnhart MD, Wilson Er, Lineberger JA, klocke NW, Lehenbauer TW (2006) Outcome and prognostic factors in non-ambulatory Hansen type I intervertebral disc extrusions: 308 cases. Vet Comp Orthop Traumatol 19:29-34.
Scott HW (1997) Hemilaminectomy for the treatment of thoracolumbar disc disease in the dog: a follow-up study of 40 cases. J Small Anim Pract 38:488-494.
Sickesz M (1986) De standsafwijking van de wervels. In: (eds: Sickesz M) klinische orthomanipulatie, 1st edn. Ankh-Hermes bv, Deventer, pp. 7-15.
Simpson ST (1992) Intervertebral disc disease. Vet Clin N Am Small Anim Pract 22:889-897.
Sukhiani HR, Parent JM, Atilola MAO, Holmberg DL (1996) Intervertebral disc disease in dogs with signs of back pain alone: 25 cases (1986-1993). J Am Vet Med Assoc 209:1275-1279.
Tanaka H, Nakamaya M, Takase k (2004) usefulness of myelography with multiple views in diagnosis of circumferential location of disc material in dogs with thoracolumbar intervertebral disc herniation. J Vet Med Sci 66:827-833.
Van de Veen EA, de Vet HC, Pool JJ, Schuller W, de Zoete A, Bouter LM (2005) Variance in manual treatment of nonspecific low back pain between orthomanual physicians, manual therapists, and chiropractors. J Manipul Physiol Ther 28:108-116.
Wheeler SJ (1995) Neurological examination of the limbs and body. In: (eds: Wheeler SJ) BSAVA Manual of small animal neurology, 2nd edn. BSAVA, Cheltenham, pp. 27-36.
Wheeler SJ, Sharp NJH (1993) Cervical disc disease. In: (eds: Wheeler SJ, Sharp NJH) Small animal spinal disorders, diagnosis and surgery, 1st edn. Mosby-Wolfe Publishers, London, pp. 85-108.